For getting help during the practical activities access: Instructions and Interpretations.
Introduction
Case-control studies are an important category of clinical studies which evaluates the link between one or more prognostic factors and various pathological conditions.
In these studies, the relationship between the factor (s) and the occurrence of a disease is assessed, or whether a particular factor changes the progression of a disease (amelioration or healing). Prognostic factors can be: risk factors (favoring the occurrence of a disease) or protective factors (prevent the disease or help the process of healing). Investigating the relationship between possible prognostic factors and disease can be achieved through all types of data collection without the researcher intervening.
In the case-control study, it starts from the fact that the disease is rare and the cases are first identified ( subjects who have a certain disease) and then the controls are chose: subjects with similar characteristics, (gender, age, socio-economic status) they do not have the disease. Only after that, with retrospective or transversal methods, the researcher is looking for prognostic factors in these groups. There are several methods for identifying prognostic factors, some of them are: laboratory determinations, standardized questionnaire, interview with the subject / family members, or consultation of medical records of subjects enrolled in the study.
Strengths of the case-control study:·Efficacy in case of rare diseases or high period of latency diseases· Relatively easy to achieve (not expensive, it takes a short period of time)· Allows analysis of several prognostic factors Weaknesses of the case-control study:can bring false information (eg. subjects forget some of previous exposures, or sick subjects tend to remember even non-significant exposures in comparison to healthy people) the time between the onset of exposure and the onset of the illness may be difficult to determine
Example of a case-control study:
An example of case-control study is the one led by the authors Destaalem Gebremedhin, Haftu Berh and Kahsu Gebrekirstos, a study entitled „Risk Factors for Neonatal Sepsis in Public Hospitals of Mekelle City, North Ethiopia,2015: Unmatched Case Control Study”,published in 2016,in the journal Plos One (ISI, IF:2.80, Q1).Article source:
http://journals.plos.org/plosone/article/file?id=10.1371/journal.pone.0154798&type=printable
Definitions used in the study:Neonatal sepsis - Systemic inflammatory response in the presence, or as a result of a proven or suspected infection in a newborn. The infection can be bacterial, viral or fungal.
Scenario-example
Gebremedhin D et al. have conducted a study on newborns registered at public hospitals in Mekelle, North Ethiopia, from December 2014 to June 2015, to identify risk factors for neonatal sepsis. The data needed for the study were collected from the infant's medical records and questionnaire applied to the mothers. Two groups of subjects were compiled in the study: a sample of 78( newborns diagnosed with neonatal sepsis) and a control group of 156 (newborns without sepsis).
The inclusion criteria for cases were:· newborns registered in pediatric or neonatal intensive care in public hospitals in Mekelle City, North Ethiopia during the study period with at least one of the following IMNCI (Integrated Management of Neonatal and Childhood Illness) clinical features: fever (≥37.5 ° C) or hypothermia (≤ 35.5 ° C), increased breathing (≥ 60 breaths per minute), severe chest retraction, movements only when stimulated, seizures, lethargy or unconsciousness · at least 2 haematological criteria: total white blood cells (12000 cells/m3), absolute neutrophil number (1500 cells/mm3 or >7500 cells/mm3), erythrocyte sedimentation rate (>15/1h), number of platelets( > 440 cells/m3) .
The inclusion criteria for the control group were:· newborns who did not meet the sepsis criteria and who were enrolled in neonatal pediatric or intensive care units of public hospitals in Mekelle, North Ethiopia during the study period
Reaserch protocol
Aim and objectives
The aim of this study was to evaluate the association between the mother's history of urinary tract infection(UTI) or sexually transmitted infection(STI) and neonatal sepsis.
Objectives: testing for the link between the factor (prolonged rupture of the membrane) and the disease (neonatal sepsis)· quantifying the importance of this link : estimation of the odds ratio indicator (OR) 2. Domain of research: risk factors or/and prognostic factors 3. Study type:· Based on study objectives: analytical study· Based on the results of the researchers: observational study· Based on the matching technique that was used in choosing the groups: without matching Note:analytical study = groups of patients are compared, associations between different clinical characteristics are testeddescriptive study=it describes a number of cases or a single group of patients and do not search for possible associations / linksobservational study = the type of study that does not involve the researcher intervention on the progression of the disease.matching in studies = for each subject in the case group/exposed group there is a subject with similar characteristics for the control /unexposed group. For ex. Matching by: same sex, similar age (identical or +/- 2 years), same risk factors (eg diabetics). The match can be made 1: 1 or 1: 2, 1: 3, 1: 4 (one case at 1/2/3/4 controls). 4. Target population and study sample: Target population: Newborn babies arriving in the world in the section of obstetrics in public hospitals in Mekelle, North Ethiopia, December 2014 - June 2015. Study sample:Inclusion criteria: Babies born between December 2014 and June 2015 in the Mekelle, Northern Ethiopia hospitals' public hospitals.Exclusion criteria :Note: In this scenario, the exclusion criteria are not mentioned.Sample size:The authors mention in the article that the size of the sample was estimated at 234 subjects for a type II error of 0,20 (a 80% study power), a proportion of UTI/STI with neonates without sepsis of 13% (determined in another study per similar population), control/cases (ratio) = 2: 1, and an OR = 2,87. We believe that the size of the sample was sufficient. 5. Data collection method· based on the studied population: systematic random sampling-see the Method section of the article· based on the duration of data collection: longitudinal retrospective· based on the grouping method: Case – control (the two groups were defined according to the presence/absence of neonatal sepsis).
!Note: Types of probabilistic samplingRandom simple sampling: Each element within the population has the same chance of being included in the sampleRandom systematic sampling: assumes random choice of a start number (step), from which, adding a fixed size will result in a unit (element) of the sampleRandom layered sampling: consists of dividing the research population into layers according to certain characteristics (genes, age groups) to randomly subtract sub-samples (groups) proportional to the size of the layer from each layer, groups that are then combined to obtain a single sampleRandom cluster sampling: The research population is divided into clusters and a number of groups are randomly selected with all the units included.
6. Statistical analysis Demographic and clinical characteristics of the mother:Age: continuous quantitative variableCivil status, religion, ethnicity, occupation: nominal qualitative variablesEducational level (primary school, secondary school, college or faculty): ordinal qualitative variableHistory of urinary tract infection or sexually transmitted infection (TUI/STI), hypertension disorders, the birth environment, prolonged membrane rupture, intrapartum fever: dichotomial qualitative variables Demographic and clinical features of the newborn:Newborn gender (M/F), Apgar score at 1minute (7 coded with 1 versus ≥7 coded with 0), Apgar score at 5 minutes (7 coded with 1 versus ≥7 coded with 0), birth resuscitation (yes/no), sepsis (yes/no): dichotomial qualitative variables
Weight at birth (1500-2500 grams, ≥2500 grams), gestational age ( 42 weeks): ordinal qualitative variables
Data description: It can be done either through a frequency table, contingency table, or vertical or horizontal column chart.
The bivariate association between the exposer factor and the disease can be demonstrated trough statistical Chi-square test or Fisher's exact test (the latter applies if expected frequencies in the contingency table are 20% of cells)
Multivariate association between the exposure factor and the disease: through multivariate logistic regression.
To quantify the importance of the association between the risk factor and the disease, the chances of having sepsis (OR) and the associated 95% confidence interval (CI) will be calculated.· If the estimated odds ratio OR> 1 and both ends (lower limit and upper limit) of the confidence interval are greater than >1 then it can be stated that the exposure factor is a risk factor· If the estimated odds ratio OR 1 and both ends (lower limit and upper limit) of the confidence interval are less than 1 then we can assume that the exposure factor can be a protective factor.· If the odds ratio OR = 1 or the confidence interval contains 1 then we can say that we do not have enough evidence to demonstrate that the exposure factor is a risk or a protective factor. !Note: The odds ratio can be determined in the unadjusted form (also referred to as the "crude odds ratio") representing the chance of disease in exposed versus non-exposed patients and / or in the adjusted form (also called the "adjusted odds ratio") representing the chance of disease in exposed versus non-exposed patients adjusted after the presence of other covariates.
Expected results. Data analysis and presentation
1. Sample description
In the present study, the description of the characteristics measured on the sample was made using the contingency tables presented in a condensed form in the Results section of the article.
Table 1. Description of the socio-demographic characteristics of the study groups
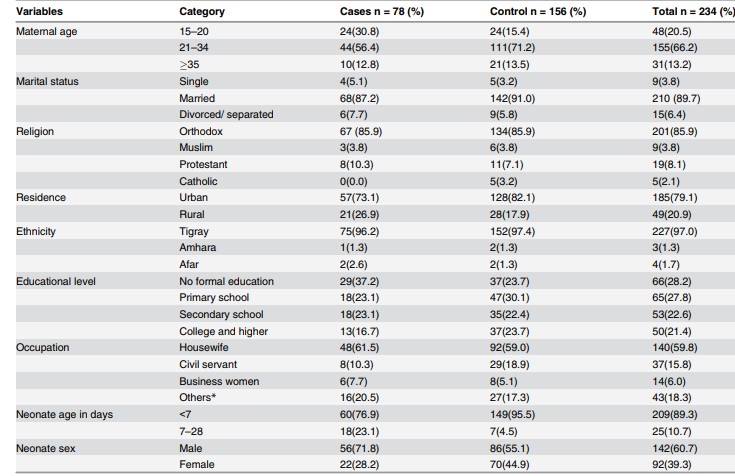
Source: imagine preluată din articolul Gebremedhin D, Berhe H, Gebrekirstos K. Risk Factors for Neonatal Sepsis in Public Hospitals of Mekelle City, North Ethiopia, 2015: Unmatched Case Control Study. PLoS One. 2016 May 10;11(5):e0154798.
- 2. Bivariate and multivariate association between a history of urinary tract infection or a sexually transmitted infection and neonatal sepsis.
The contingency of risk factor (TUI / STI) and disease (sepsis):
|
Sepsis(yes) |
Sepsis (no) |
Total |
|
|
AIU/AIS (yes) |
40 |
21 |
61 |
|
AIU/AIS(no) |
38 |
135 |
173 |
|
Total |
78 |
156 |
234 |
TUI / STI= the history of the mother of urinary infection or sexually transmitted infection
Table 2. Bivariate and multivariate association between TUI /STI and neonatal sepsis
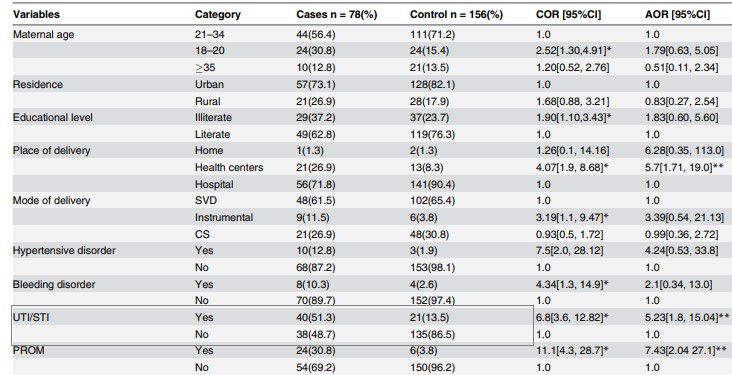
Source: preluat din articolul Gebremedhin D, Berhe H, Gebrekirstos K. Risk Factors for Neonatal Sepsis in Public Hospitals of Mekelle City, North Ethiopia, 2015: Unmatched Case Control Study. PLoS One. 2016 May 10;11(5):e0154798.
Column Chart for the relationship between risk factor and disease (made in Excel):
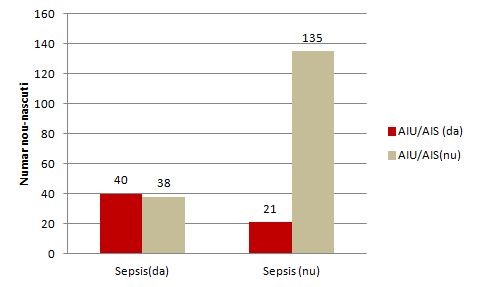
Figure 1. Relationship between the TUI(AIU) /STI( AIS) mother's history and neonatal sepsis
The odds ratio (unadjusted OR) and the associated confidence interval (Table 2)( to calculate the point estimator and associated confidence interval the EpiInfo program can be used) the result writing format: point estimator (95% CI: superior limit-inferior limit); OR = 6,8 (95% CI: 3,6-12,8)
The value of p (made in EpiInfo is reported as p = value - the name of the test used, with a maximum of 3 decimal places, if p 0.001 then "p 0.001"): p 0.001-Chi-square test
Note: In the present study, the authors also estimated the rate of chance adjusted for different associations such as maternal age, background, type of birth, prolonged rupture of the membrane, etc.
Interpretation of data. Discussions
1. Interpreting the results from a statistical point o view: The aim of the study was to test and quantify the association between the mother's history of UTI /STI and neonatal sepsis.From a statistical point of view, association testing was made by formulating two hypotheses (the null hypothesis and the alternative hypothesis), the null hypothesis being the one tested. In the case of its rejection, we affirm that we are in favor of the alternative hypothesis.Null Hypothesis: There is no significant association between the mother's history of UTI /STI and neonatal sepsis.Alternative hypothesis: There is a significant association between the mother's history of UTI /STI and neonatal sepsis.Because p 0.05, there is significant association between the mother's history of UTI /STI and neonatal sepsis.From a statistical point of view, the quantification of the association was achieved by punctual estimation of the OR indicator (unadjusted and adjusted) and the 95% confidence interval associated with it. Point estimate:OR = 6,8; newborns whose mothers had a history of UTI/STI during pregnancy had a 6,8-times higher chance of developing sepsis compared to neonates whose mothers did not have UTI/STI.Reliable interval for OR: 95% CI: 3,6-12,8 - we are 95% sure that the odds ratio for the study population will be between 3,6 and 12,8 (if we extract samples of the same size from the study population, 95% of CI will contain the chance rate in the target population of newborns).In addition, OR> 1 and both ends of the confidence interval were greater than 1, so the history of the UTI/STI mother is a risk factor for neonatal sepsis. !Note: In the study presented, the authors also analyzed the association between UTI/STI in the context of presence of other covariates (maternal and neonatal factors) such as hypertension in pregnancy, intrapartum fever, birth complications, low score at 5 minutes, etc. - and an adjusted OR = 5.23, 95% IC: 1,8-15,04. Since the results were statistically significant, it can be argued that the history of UTI / STI of the mother remains an independent risk factor for neonatal sepsis.
2. Interpreting the results from a clinical point of view: The size of the OR indicator in clinical context:• Very important / moderate / not importantOR = 6,8 shows a moderate magnitude in the context of similar studies performed on the same population as having an OR = 12,9 (see section Discussion of the article)
Accuracy of result (see confidence interval): • Relatively accurate / imprecise (broad range - imprecise results, narrow range - accurate results)The confidence interval of 95%: 3,6-12,8 can be considered as a relatively accurate interval • Relatively important clinical connection (both ends with significant clinical value) The link between the maternal factors (UTI / STI history during pregnancy) can be considered as clinically relevant and relatively important (in the simulation of the size of the sample, the authors considering an OR = 2,87 effect size to be of interest).
Conclusions of the study
The history of the mother`s urinary tract infection or sexually transmitted infection as a maternal factor contributes to the risk of neonatal sepsis, being even an independent risk factor.
On Infomed server, in your own folder make a new LAB02 folder. Download the following file in this folder and answer inside to all practical activities requiered. Save the file.
Results obtained with EpiInfo - for those who cannot install it
For getting help during the practical activities access: Instructions and Interpretations.
The Bibliographic Study & Citing References Using the Vancouver Style
The bibliographic study is the study of the specialized literature by consulting scientific articles, papers, specialized books and treatises on the subject that you intend to research. It is an essential stage in the preparation and construction of a research, as well as in the communication of its results.
Once the research idea has been established, the following step is bibliographic documentation. The identification data of scientific articles, papers, specialized books and treatises (authors, title ...) together with the notes and / or the summary of the article constitute a bibliographic record. This helps you easily retrieve information from articles or books that you find of interest when documenting your future research. The bibliographic records will later be used to justify the information presented in your work, by quoting the corresponding scientific works, as well as to build the reference list that you will write in the "References" section, arranged in the order in which they have been quoted in your work.
Bibliographic documentation is performed by accessing various sources such as: the personal library, the University Library, the Electronic Library of UMF Cluj (http://www.umfcluj.ro/educatie-ro/reurseedu-ro/biblioteca-ro), specialty journals (PubMed: www.pubmed.gov, SpringerLink: www.springerlink.com, ScienceDirect: www.sciencedirect.com, ProQuest, Oxford Journals, BMJ Journals, Thieme, Medline, Thomson Micromedex, The Cochrane Collaboration: http://www.cochranelibrary.com, Dentistry and Oral Sciences (DOSS): https://health.ebsco.com/products/dentistry-oral-sciences-source, EMBASE: www.embase.com, etc.).
PubMed (https://www.ncbi.nlm.nih.gov/pubmed), the database of the National Medicine Library of the United States of America, is a free resource that provides access to MEDLINE, quotes and abstracts in the fields of medicine (general, dental, veterinary), nursing, and life sciences. PubMed allows searching for specialized articles by providing access to their abstracts. For some of these articles it also provides access to their full-text or links to the publishing journal. The search terms used by PubMed are those of the MeSH (Medical Subject Headings), a standard dictionary of medical terms. For more details regarding MeSH, you can access the following resource: https://www.nlm.nih.gov/bsd/disted/video.
A PubMed search allows you to identify the following pieces of information:
- Title of the article
- Authors' names
- Abbreviation of the journal, year of publication, volume, issue, extreme pages
- Language in which the article was published (if not English)
- Searching with PubMed also allows certain restrictions to be imposed, regarding the:
- Article type (review, editorial, original article, clinical trial, etc.)
- Type of access (abstract / full text - link to the journal where the article was published / free full text - access to Open Access articles)
- Publication period
- Species (human / animal subjects)
- Other additional filters (the language in which the article was published, demographic criteria: age, gender).
Quoting references in Vancouver style
If you use information from published sources in your texts, or cite a full or partial text, you must clearly state this. By using a reference, you credit the authors of that source and defend yourself from possible accusations of plagiarism or pertaining to the truthfulness and accuracy of the respective information. A reference links your text to the original source of the information.
The reference citation style in the medical field uses the Vancouver system.
Its use implies:
signaling the use of a reference in the text by inserting numbers between straight brackets. These numbers will be in ascending order, starting from the beginning of the document.
the existence of a numbered references list at the end of the document, containing the details of the sources quoted in the text. These details will be written in a standard format, specific to each type of quoted document (article published in a printed magazine, article published on the internet, book, book chapter, etc.)

Example of Vancouver style citations
Formatting the reference list - punctuation marks, separators between entities, etc. - depends on the type of quoted document.
References for original articles published in printed journals
General structure:
Name of author(s) followed by first name(s) initial(s)1. Article title. Abbreviated name of the journal2. year of publication;volume number(issue number)3:extreme pages4.
1 For the initial / initials of the surname, no spaces or punctuation marks are used between the initials, the authors are separated by commas (,) and if there are 7 or more authors, only the first 6 authors are mentioned, followed by the expression: , et al.
2 To find the abbreviation of a journal, you can use the following link: https://www.ncbi.nlm.nih.gov/nlmcatalog/journals
3 the volume number usually corresponds to the number of appearances of the journal since its launch and is followed, between round brackets, by the issue number inside that volume
4 The first and last pages of the article, while omitting all repeating digits in the last page.
Example:
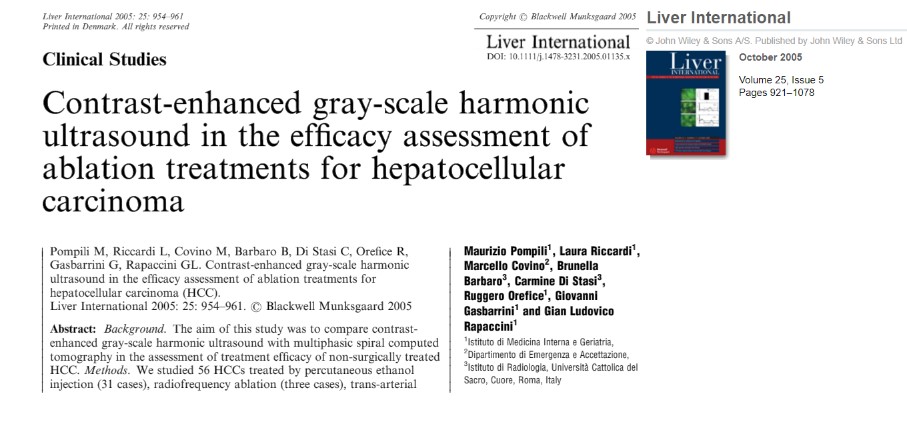
The Reference: Pompili M, Riccardi L, Covino M, Barbaro B, Di Stasi C, Orefice R, et al. Contrastenhanced gray-scale harmonic ultrasound in the efficacy assessment of ablation treatments for hepatocellular carcinoma. Liver Int. 2005;25(5):954-61.
References for original articles published in on-line journals, only in electronic format
General structure:
Author(s)1. Article title. Abbreviated name of the journal [Internet] year of publication [cited citation date2]; volume number(issue number):article identifier. Available from: web page address.
1 For the initial / initials of the surname, no spaces or punctuation marks are used between the initials, the authors are separated by commas (,) and if there are 7 or more authors, only the first 6 authors are mentioned, followed by the expression: , et al.
2 The current date, when you have consulted and cited the article
Optionally, if available, you can add electronic identification information: Digital Object Identifier (DOI) - useful for easy Internet search, PubMed ID - unique ID number in the Medline / Pubmed database, or PubMed Central PMCID: doi: zzzzzzz. PubMed PMID: zzzzzzz; PubMed Central PMCID: PMC zzzzzzz.
Example of a reference to an original article from a journal published in electronic format:

The reference: Dudea D, Lasserre JF, Alb C, Culic B, Pop Ciutrila IS, Colosi H. Patients' perspective on dental aesthetics in a South-Eastern European community. J Dent [Internet] 2012 [cited 2018 Mar 2];40 Suppl 1:e72-81. DOI: 10.1016/j.jdent.2012.01.016. Available from: https://www.sciencedirect.com/science/article/pii/S0300571212000255?via%3Dihub
References for books
General structure:
Author(s)1. Book title. Edition2. Publication place3: Publisher; year of publication.
1 For the initial / initials of the surname, no spaces or punctuation marks are used between the initials, the authors are separated by commas (,) and if there are 7 or more authors, only the first 6 authors are mentioned, followed by the expression: , et al.
2 If the book is at its first edition, this piece of information is omitted
3 The city in which the publisher is based
As a rule, all the information required to build the reference is contained on the cover and on the first pages of the book.
Example:

Eisen HN. Immunology: an introduction to molecular and cellular principles of the immune response. 5th ed. New York: Harper and Row; 1974.
More details on the Vancouver citation system can be found at: https://www.nlm.nih.gov/bsd/uniform_requirements.html
Practical activity
Aims and benefits of this practical activit:
- Performing a scenario based search using the PICO method.
- Creating a search strategy to identify a manageable number of articles on a certain research topic.
- Using Clinical Queries and search restrictions by certain criteria offered in PubMed (e.g. by article type, year of publication, etc.).
- Acquiring the skills for writing references in the Vancouver style standard.
- Creating a bibliographic record file with a table of contents, to easily retrieve items of interest by title.
- Aquiring bibliographic documentation skills for research and current practice.
Clinical Queries allows the search for clinical studies according to their type: therapy, diagnostic, prognostic (risk factors).
On Infomed server, in your own folder make a new LAB01 folder. Download the following file in this folder and answer inside to all practical activities requiered. Save the file.
Ussefull web links:
- PICO Query: https://pubmedhh.nlm.nih.gov/nlmd/pico/piconew.php
- Pubmed Clinical Query: https://www.ncbi.nlm.nih.gov/pubmed/clinical
For getting help during the practical activities access: Instructions and Interpretations.
The Bibliographic Study & Citing References Using the Vancouver Style
The bibliographic study is the study of the specialized literature by consulting scientific articles, papers, specialized books and treatises on the subject that you intend to research. It is an essential stage in the preparation and construction of a research, as well as in the communication of its results.
Once the research idea has been established, the following step is bibliographic documentation. The identification data of scientific articles, papers, specialized books and treatises (authors, title ...) together with the notes and / or the summary of the article constitute a bibliographic record. This helps you easily retrieve information from articles or books that you find of interest when documenting your future research. The bibliographic records will later be used to justify the information presented in your work, by quoting the corresponding scientific works, as well as to build the reference list that you will write in the "References" section, arranged in the order in which they have been quoted in your work.
Bibliographic documentation is performed by accessing various sources such as: the personal library, the University Library, the Electronic Library of UMF Cluj (http://www.umfcluj.ro/educatie-ro/reurseedu-ro/biblioteca-ro), specialty journals (PubMed: www.pubmed.gov, SpringerLink: www.springerlink.com, ScienceDirect: www.sciencedirect.com, ProQuest, Oxford Journals, BMJ Journals, Thieme, Medline, Thomson Micromedex, The Cochrane Collaboration: http://www.cochranelibrary.com, Dentistry and Oral Sciences (DOSS): https://health.ebsco.com/products/dentistry-oral-sciences-source, EMBASE: www.embase.com, etc.).
PubMed (https://www.ncbi.nlm.nih.gov/pubmed), the database of the National Medicine Library of the United States of America, is a free resource that provides access to MEDLINE, quotes and abstracts in the fields of medicine (general, dental, veterinary), nursing, and life sciences. PubMed allows searching for specialized articles by providing access to their abstracts. For some of these articles it also provides access to their full-text or links to the publishing journal. The search terms used by PubMed are those of the MeSH (Medical Subject Headings), a standard dictionary of medical terms. For more details regarding MeSH, you can access the following resource: https://www.nlm.nih.gov/bsd/disted/video.
A PubMed search allows you to identify the following pieces of information:
- Title of the article
- Authors' names
- Abbreviation of the journal, year of publication, volume, issue, extreme pages
- Language in which the article was published (if not English)
- Searching with PubMed also allows certain restrictions to be imposed, regarding the:
- Article type (review, editorial, original article, clinical trial, etc.)
- Type of access (abstract / full text - link to the journal where the article was published / free full text - access to Open Access articles)
- Publication period
- Species (human / animal subjects)
- Other additional filters (the language in which the article was published, demographic criteria: age, gender).
Quoting references in Vancouver style
If you use information from published sources in your texts, or cite a full or partial text, you must clearly state this. By using a reference, you credit the authors of that source and defend yourself from possible accusations of plagiarism or pertaining to the truthfulness and accuracy of the respective information. A reference links your text to the original source of the information.
The reference citation style in the medical field uses the Vancouver system.
Its use implies:
signaling the use of a reference in the text by inserting numbers between straight brackets. These numbers will be in ascending order, starting from the beginning of the document.
the existence of a numbered references list at the end of the document, containing the details of the sources quoted in the text. These details will be written in a standard format, specific to each type of quoted document (article published in a printed magazine, article published on the internet, book, book chapter, etc.)

Example of Vancouver style citations
Formatting the reference list - punctuation marks, separators between entities, etc. - depends on the type of quoted document.
References for original articles published in printed journals
General structure:
Name of author(s) followed by first name(s) initial(s)1. Article title. Abbreviated name of the journal2. year of publication;volume number(issue number)3:extreme pages4.
1 For the initial / initials of the surname, no spaces or punctuation marks are used between the initials, the authors are separated by commas (,) and if there are 7 or more authors, only the first 6 authors are mentioned, followed by the expression: , et al.
2 To find the abbreviation of a journal, you can use the following link: https://www.ncbi.nlm.nih.gov/nlmcatalog/journals
3 the volume number usually corresponds to the number of appearances of the journal since its launch and is followed, between round brackets, by the issue number inside that volume
4 The first and last pages of the article, while omitting all repeating digits in the last page.
Example:

The Reference: Pompili M, Riccardi L, Covino M, Barbaro B, Di Stasi C, Orefice R, et al. Contrastenhanced gray-scale harmonic ultrasound in the efficacy assessment of ablation treatments for hepatocellular carcinoma. Liver Int. 2005;25(5):954-61.
References for original articles published in on-line journals, only in electronic format
General structure:
Author(s)1. Article title. Abbreviated name of the journal [Internet] year of publication [cited citation date2]; volume number(issue number):article identifier. Available from: web page address.
1 For the initial / initials of the surname, no spaces or punctuation marks are used between the initials, the authors are separated by commas (,) and if there are 7 or more authors, only the first 6 authors are mentioned, followed by the expression: , et al.
2 The current date, when you have consulted and cited the article
Optionally, if available, you can add electronic identification information: Digital Object Identifier (DOI) - useful for easy Internet search, PubMed ID - unique ID number in the Medline / Pubmed database, or PubMed Central PMCID: doi: zzzzzzz. PubMed PMID: zzzzzzz; PubMed Central PMCID: PMC zzzzzzz.
Example of a reference to an original article from a journal published in electronic format:

The reference: Dudea D, Lasserre JF, Alb C, Culic B, Pop Ciutrila IS, Colosi H. Patients' perspective on dental aesthetics in a South-Eastern European community. J Dent [Internet] 2012 [cited 2018 Mar 2];40 Suppl 1:e72-81. DOI: 10.1016/j.jdent.2012.01.016. Available from: https://www.sciencedirect.com/science/article/pii/S0300571212000255?via%3Dihub
References for books
General structure:
Author(s)1. Book title. Edition2. Publication place3: Publisher; year of publication.
1 For the initial / initials of the surname, no spaces or punctuation marks are used between the initials, the authors are separated by commas (,) and if there are 7 or more authors, only the first 6 authors are mentioned, followed by the expression: , et al.
2 If the book is at its first edition, this piece of information is omitted
3 The city in which the publisher is based
As a rule, all the information required to build the reference is contained on the cover and on the first pages of the book.
Example:

Eisen HN. Immunology: an introduction to molecular and cellular principles of the immune response. 5th ed. New York: Harper and Row; 1974.
More details on the Vancouver citation system can be found at: https://www.nlm.nih.gov/bsd/uniform_requirements.html
Practical activity
Aims and benefits of this practical activit:
- Performing a scenario based search using the PICO method.
- Creating a search strategy to identify a manageable number of articles on a certain research topic.
- Using Clinical Queries and search restrictions by certain criteria offered in PubMed (e.g. by article type, year of publication, etc.).
- Acquiring the skills for writing references in the Vancouver style standard.
- Creating a bibliographic record file with a table of contents, to easily retrieve items of interest by title.
- Aquiring bibliographic documentation skills for research and current practice.
Clinical Queries allows the search for clinical studies according to their type: therapy, diagnostic, prognostic (risk factors).
On Infomed server, in your own folder make a new LAB01 folder. Download the following file in this folder and answer inside to all practical activities requiered. Save the file.
Ussefull web links:
- PICO Query: https://pubmedhh.nlm.nih.gov/nlmd/pico/piconew.php
- Pubmed Clinical Query: https://www.ncbi.nlm.nih.gov/pubmed/clinical
For getting help during the practical activities access: Instructions and Interpretations.
Introduction
Case-control studies are an important category of clinical studies which evaluates the link between one or more prognostic factors and various pathological conditions.
In these studies, the relationship between the factor (s) and the occurrence of a disease is assessed, or whether a particular factor changes the progression of a disease (amelioration or healing). Prognostic factors can be: risk factors (favoring the occurrence of a disease) or protective factors (prevent the disease or help the process of healing). Investigating the relationship between possible prognostic factors and disease can be achieved through all types of data collection without the researcher intervening.
In the case-control study, it starts from the fact that the disease is rare and the cases are first identified ( subjects who have a certain disease) and then the controls are chose: subjects with similar characteristics, (gender, age, socio-economic status) they do not have the disease. Only after that, with retrospective or transversal methods, the researcher is looking for prognostic factors in these groups. There are several methods for identifying prognostic factors, some of them are: laboratory determinations, standardized questionnaire, interview with the subject / family members, or consultation of medical records of subjects enrolled in the study.
Strengths of the case-control study:·Efficacy in case of rare diseases or high period of latency diseases· Relatively easy to achieve (not expensive, it takes a short period of time)· Allows analysis of several prognostic factors Weaknesses of the case-control study:can bring false information (eg. subjects forget some of previous exposures, or sick subjects tend to remember even non-significant exposures in comparison to healthy people) the time between the onset of exposure and the onset of the illness may be difficult to determine
Example of a case-control study:
An example of case-control study is the one led by the authors Destaalem Gebremedhin, Haftu Berh and Kahsu Gebrekirstos, a study entitled „Risk Factors for Neonatal Sepsis in Public Hospitals of Mekelle City, North Ethiopia,2015: Unmatched Case Control Study”,published in 2016,in the journal Plos One (ISI, IF:2.80, Q1).Article source:
http://journals.plos.org/plosone/article/file?id=10.1371/journal.pone.0154798&type=printable
Definitions used in the study:Neonatal sepsis - Systemic inflammatory response in the presence, or as a result of a proven or suspected infection in a newborn. The infection can be bacterial, viral or fungal.
Scenario-example
Gebremedhin D et al. have conducted a study on newborns registered at public hospitals in Mekelle, North Ethiopia, from December 2014 to June 2015, to identify risk factors for neonatal sepsis. The data needed for the study were collected from the infant's medical records and questionnaire applied to the mothers. Two groups of subjects were compiled in the study: a sample of 78( newborns diagnosed with neonatal sepsis) and a control group of 156 (newborns without sepsis).
The inclusion criteria for cases were:· newborns registered in pediatric or neonatal intensive care in public hospitals in Mekelle City, North Ethiopia during the study period with at least one of the following IMNCI (Integrated Management of Neonatal and Childhood Illness) clinical features: fever (≥37.5 ° C) or hypothermia (≤ 35.5 ° C), increased breathing (≥ 60 breaths per minute), severe chest retraction, movements only when stimulated, seizures, lethargy or unconsciousness · at least 2 haematological criteria: total white blood cells (12000 cells/m3), absolute neutrophil number (1500 cells/mm3 or >7500 cells/mm3), erythrocyte sedimentation rate (>15/1h), number of platelets( > 440 cells/m3) .
The inclusion criteria for the control group were:· newborns who did not meet the sepsis criteria and who were enrolled in neonatal pediatric or intensive care units of public hospitals in Mekelle, North Ethiopia during the study period
Reaserch protocol
Aim and objectives
The aim of this study was to evaluate the association between the mother's history of urinary tract infection(UTI) or sexually transmitted infection(STI) and neonatal sepsis.
Objectives: testing for the link between the factor (prolonged rupture of the membrane) and the disease (neonatal sepsis)· quantifying the importance of this link : estimation of the odds ratio indicator (OR) 2. Domain of research: risk factors or/and prognostic factors 3. Study type:· Based on study objectives: analytical study· Based on the results of the researchers: observational study· Based on the matching technique that was used in choosing the groups: without matching Note:analytical study = groups of patients are compared, associations between different clinical characteristics are testeddescriptive study=it describes a number of cases or a single group of patients and do not search for possible associations / linksobservational study = the type of study that does not involve the researcher intervention on the progression of the disease.matching in studies = for each subject in the case group/exposed group there is a subject with similar characteristics for the control /unexposed group. For ex. Matching by: same sex, similar age (identical or +/- 2 years), same risk factors (eg diabetics). The match can be made 1: 1 or 1: 2, 1: 3, 1: 4 (one case at 1/2/3/4 controls). 4. Target population and study sample: Target population: Newborn babies arriving in the world in the section of obstetrics in public hospitals in Mekelle, North Ethiopia, December 2014 - June 2015. Study sample:Inclusion criteria: Babies born between December 2014 and June 2015 in the Mekelle, Northern Ethiopia hospitals' public hospitals.Exclusion criteria :Note: In this scenario, the exclusion criteria are not mentioned.Sample size:The authors mention in the article that the size of the sample was estimated at 234 subjects for a type II error of 0,20 (a 80% study power), a proportion of UTI/STI with neonates without sepsis of 13% (determined in another study per similar population), control/cases (ratio) = 2: 1, and an OR = 2,87. We believe that the size of the sample was sufficient. 5. Data collection method· based on the studied population: systematic random sampling-see the Method section of the article· based on the duration of data collection: longitudinal retrospective· based on the grouping method: Case – control (the two groups were defined according to the presence/absence of neonatal sepsis).
!Note: Types of probabilistic samplingRandom simple sampling: Each element within the population has the same chance of being included in the sampleRandom systematic sampling: assumes random choice of a start number (step), from which, adding a fixed size will result in a unit (element) of the sampleRandom layered sampling: consists of dividing the research population into layers according to certain characteristics (genes, age groups) to randomly subtract sub-samples (groups) proportional to the size of the layer from each layer, groups that are then combined to obtain a single sampleRandom cluster sampling: The research population is divided into clusters and a number of groups are randomly selected with all the units included.
6. Statistical analysis Demographic and clinical characteristics of the mother:Age: continuous quantitative variableCivil status, religion, ethnicity, occupation: nominal qualitative variablesEducational level (primary school, secondary school, college or faculty): ordinal qualitative variableHistory of urinary tract infection or sexually transmitted infection (TUI/STI), hypertension disorders, the birth environment, prolonged membrane rupture, intrapartum fever: dichotomial qualitative variables Demographic and clinical features of the newborn:Newborn gender (M/F), Apgar score at 1minute (7 coded with 1 versus ≥7 coded with 0), Apgar score at 5 minutes (7 coded with 1 versus ≥7 coded with 0), birth resuscitation (yes/no), sepsis (yes/no): dichotomial qualitative variables
Weight at birth (1500-2500 grams, ≥2500 grams), gestational age ( 42 weeks): ordinal qualitative variables
Data description: It can be done either through a frequency table, contingency table, or vertical or horizontal column chart.
The bivariate association between the exposer factor and the disease can be demonstrated trough statistical Chi-square test or Fisher's exact test (the latter applies if expected frequencies in the contingency table are 20% of cells)
Multivariate association between the exposure factor and the disease: through multivariate logistic regression.
To quantify the importance of the association between the risk factor and the disease, the chances of having sepsis (OR) and the associated 95% confidence interval (CI) will be calculated.· If the estimated odds ratio OR> 1 and both ends (lower limit and upper limit) of the confidence interval are greater than >1 then it can be stated that the exposure factor is a risk factor· If the estimated odds ratio OR 1 and both ends (lower limit and upper limit) of the confidence interval are less than 1 then we can assume that the exposure factor can be a protective factor.· If the odds ratio OR = 1 or the confidence interval contains 1 then we can say that we do not have enough evidence to demonstrate that the exposure factor is a risk or a protective factor. !Note: The odds ratio can be determined in the unadjusted form (also referred to as the "crude odds ratio") representing the chance of disease in exposed versus non-exposed patients and / or in the adjusted form (also called the "adjusted odds ratio") representing the chance of disease in exposed versus non-exposed patients adjusted after the presence of other covariates.
Expected results. Data analysis and presentation
1. Sample description
In the present study, the description of the characteristics measured on the sample was made using the contingency tables presented in a condensed form in the Results section of the article.
Table 1. Description of the socio-demographic characteristics of the study groups

Source: imagine preluată din articolul Gebremedhin D, Berhe H, Gebrekirstos K. Risk Factors for Neonatal Sepsis in Public Hospitals of Mekelle City, North Ethiopia, 2015: Unmatched Case Control Study. PLoS One. 2016 May 10;11(5):e0154798.
- 2. Bivariate and multivariate association between a history of urinary tract infection or a sexually transmitted infection and neonatal sepsis.
The contingency of risk factor (TUI / STI) and disease (sepsis):
|
Sepsis(yes) |
Sepsis (no) |
Total |
|
|
AIU/AIS (yes) |
40 |
21 |
61 |
|
AIU/AIS(no) |
38 |
135 |
173 |
|
Total |
78 |
156 |
234 |
TUI / STI= the history of the mother of urinary infection or sexually transmitted infection
Table 2. Bivariate and multivariate association between TUI /STI and neonatal sepsis

Source: preluat din articolul Gebremedhin D, Berhe H, Gebrekirstos K. Risk Factors for Neonatal Sepsis in Public Hospitals of Mekelle City, North Ethiopia, 2015: Unmatched Case Control Study. PLoS One. 2016 May 10;11(5):e0154798.
Column Chart for the relationship between risk factor and disease (made in Excel):

Figure 1. Relationship between the TUI(AIU) /STI( AIS) mother's history and neonatal sepsis
The odds ratio (unadjusted OR) and the associated confidence interval (Table 2)( to calculate the point estimator and associated confidence interval the EpiInfo program can be used) the result writing format: point estimator (95% CI: superior limit-inferior limit); OR = 6,8 (95% CI: 3,6-12,8)
The value of p (made in EpiInfo is reported as p = value - the name of the test used, with a maximum of 3 decimal places, if p 0.001 then "p 0.001"): p 0.001-Chi-square test
Note: In the present study, the authors also estimated the rate of chance adjusted for different associations such as maternal age, background, type of birth, prolonged rupture of the membrane, etc.
Interpretation of data. Discussions
1. Interpreting the results from a statistical point o view: The aim of the study was to test and quantify the association between the mother's history of UTI /STI and neonatal sepsis.From a statistical point of view, association testing was made by formulating two hypotheses (the null hypothesis and the alternative hypothesis), the null hypothesis being the one tested. In the case of its rejection, we affirm that we are in favor of the alternative hypothesis.Null Hypothesis: There is no significant association between the mother's history of UTI /STI and neonatal sepsis.Alternative hypothesis: There is a significant association between the mother's history of UTI /STI and neonatal sepsis.Because p 0.05, there is significant association between the mother's history of UTI /STI and neonatal sepsis.From a statistical point of view, the quantification of the association was achieved by punctual estimation of the OR indicator (unadjusted and adjusted) and the 95% confidence interval associated with it. Point estimate:OR = 6,8; newborns whose mothers had a history of UTI/STI during pregnancy had a 6,8-times higher chance of developing sepsis compared to neonates whose mothers did not have UTI/STI.Reliable interval for OR: 95% CI: 3,6-12,8 - we are 95% sure that the odds ratio for the study population will be between 3,6 and 12,8 (if we extract samples of the same size from the study population, 95% of CI will contain the chance rate in the target population of newborns).In addition, OR> 1 and both ends of the confidence interval were greater than 1, so the history of the UTI/STI mother is a risk factor for neonatal sepsis. !Note: In the study presented, the authors also analyzed the association between UTI/STI in the context of presence of other covariates (maternal and neonatal factors) such as hypertension in pregnancy, intrapartum fever, birth complications, low score at 5 minutes, etc. - and an adjusted OR = 5.23, 95% IC: 1,8-15,04. Since the results were statistically significant, it can be argued that the history of UTI / STI of the mother remains an independent risk factor for neonatal sepsis.
2. Interpreting the results from a clinical point of view: The size of the OR indicator in clinical context:• Very important / moderate / not importantOR = 6,8 shows a moderate magnitude in the context of similar studies performed on the same population as having an OR = 12,9 (see section Discussion of the article)
Accuracy of result (see confidence interval): • Relatively accurate / imprecise (broad range - imprecise results, narrow range - accurate results)The confidence interval of 95%: 3,6-12,8 can be considered as a relatively accurate interval • Relatively important clinical connection (both ends with significant clinical value) The link between the maternal factors (UTI / STI history during pregnancy) can be considered as clinically relevant and relatively important (in the simulation of the size of the sample, the authors considering an OR = 2,87 effect size to be of interest).
Conclusions of the study
The history of the mother`s urinary tract infection or sexually transmitted infection as a maternal factor contributes to the risk of neonatal sepsis, being even an independent risk factor.
On Infomed server, in your own folder make a new LAB02 folder. Download the following file in this folder and answer inside to all practical activities requiered. Save the file.
Results obtained with EpiInfo - for those who cannot install it
Clinical Skills Book - VARIANT 1 (NEW BOOK)
10. Critical appraisal (Evidence Based Medicine - EBM) / Efficient use of resources
10.1. Knows the systematic search methods in bibliographic databases
(Metodologia cercetării ştiinţifice)
|
Nr |
Data |
Pacient |
Diagnostic și descriere sintetică |
Spital |
Coordonator |
|
10.1.1 |
Date of Week 2 lab |
Virtual |
Applies systematic search strategies in medical databases, using the PICO technique. |
Medical Informatics - MCS |
3 |
10.2. Critically evaluates data, information, and scientific evidence in medical and academic situations (Metodologia cercetării ştiinţifice)
|
Nr |
Data |
Pacient |
Diagnostic și descriere sintetică |
Spital |
Coordonator |
|
10.2.1 |
Dates interval: Weeks 9-13 labs |
Virtual |
Classifies the types of studies in the hierarchy of medical evidence. Identifies and discusses the influence of random error, systematic error (selection, information and analysis bias), and confounders in diagnostic, therapeutic, and etiology/prognostic studies. Critically evaluates the results of a meta-analysis. Critically appraises the quality of writing in medical science articles. |
Medical Informatics - MCS |
8 |
10.3.1 Critically evaluates data found in literature in order to assess the benefits and risks of current and proposed methods of investigation, treatment and prevention of illness (Metodologia cercetării ştiinţifice)
|
Nr |
Data |
Pacient |
Diagnostic și descriere sintetică |
Spital |
Coordonator |
|
10.3.1 |
Dates interval: Weeks 2-13 labs |
Virtual |
Critically appraises diagnostic, therapeutic, and etiology/prognostic studies, in order to assess the benefits and risks of current and proposed methods of investigation, treatment and prevention of illness. Recognizes and evaluates the influence of random error, systematic error (selection, information and analysis bias), and confounders in diagnostic, therapeutic, and etiology/prognostic studies. Critically evaluates the results of meta-analyses. |
Medical Informatics - MCS |
8 |
10.4. Uses the computer for information, patient data recording and communication
(Metodologia cercetării ştiinţifice)
|
Nr |
Data |
Pacient |
Diagnostic și descriere sintetică |
Spital |
Coordonator |
|
10.4.1 |
Dates interval: Weeks 2-6 labs |
Virtual |
Records the results of a bibliographic documentation using bibliographic records and references in Vancouver style. Records the medical protocol, and analyzes clinical patient data according to this research protocol, then communicates the results of the study, observing the rules of scientific medical writing. |
Medical Informatics - MCS |
10 |
10.5. Prepares oral presentations for scientific symposia
(Metodologia cercetării ştiinţifice)
|
Nr |
Data |
Pacient |
Diagnostic și descriere sintetică |
Spital |
Coordonator |
|
10.5.1 |
Date of Week 7 lab |
Virtual |
Produces oral presentations using PowerPoint, while respecting the form, structure and content required of scientific presentations. |
Medical Informatics - MCS |
12 |
10.6. Prepares presentations for health education of the general public
(Metodologia cercetării ştiinţifice)
|
Nr |
Data |
Pacient |
Diagnostic și descriere sintetică |
Spital |
Coordonator |
|
10.6.1 |
Dates interval: Weeks 2-13 labs |
Virtual |
Presentation of medical evidence using means and language accessible to the general public. |
Medical Informatics - MCS |
12 |
Clinical Skills Book - VARIANT 2 (OLD BOOK)
8. Managerial Skills
8.1. Carries out a critical evaluation of diagnostic and screening tests
|
No. |
Date |
Patient |
Diagnosis and synthetic description |
Hospital |
Coordinator |
|
8.1.1 |
Date of Week 5 lab |
Virtual |
Calculated and interpreted diagnostic medical indicators |
Medical Informatics - MCS |
1 |
|
8.1.2 |
Date of Week 12 lab |
Virtual |
Critically appraised the validity of a diagnostic study |
Medical Informatics - MCS |
2 |
10. Critical appraisal (EBM)
10.1. Performs a critical assessment of data, information, and documents
|
No. |
Date |
Patient |
Diagnosis and synthetic description |
Hospital |
Coordinator |
|
10.1.1 |
Date of Week 12 lab |
Virtual |
Recognized selection, information and analysis bias, confounders and random error in medical studies |
Medical Informatics - MCS |
3 |
|
10.1.2 |
Date of Week 9 lab |
Virtual |
Classified types of studies in the hierarchy of evidence |
Medical Informatics - MCS |
4 |
|
10.1.3 |
Date of Week 12 lab |
Virtual |
Critically appraised studies found in literature (therapeutic, diagnostic, prognostic) |
Medical Informatics - MCS |
5 |
|
10.1.4 |
Date of Week 10 lab |
Virtual |
Critically appraised article writing. Identified errors in graphs and tables |
Medical Informatics - MCS |
6 |
|
10.1.5 |
Date of Week 9 lab |
Virtual |
Critically appraised results from a meta-analysis |
Medical Informatics - MCS |
7 |
10.2. Performs a critical evaluation of literature data to assess the benefits and risks of prophylactic, diagnostic and therapeutic measures
|
No. |
Date |
Patient |
Diagnosis and synthetic description |
Hospital |
Coordinator |
|
10.2 .1 |
Date of Week 6 lab |
Virtual |
Calculated medical indicators of therapeutic efficacy + their statistical and clinical interpretation |
Medical Informatics - MCS |
8 |
|
10.2 .2 |
Date of Week 4 lab |
Virtual |
Tested the existence of a link and quantified the importance of medical risk indicators / between prognostic factors and diseases |
Medical Informatics - MCS |
9 |
10.3. Uses the computer for bibliographic documentation, to record patient data and to communicate
|
No. |
Date |
Patient |
Diagnosis and synthetic description |
Hospital |
Coordinator |
|
10 3.1 |
Date of Week 2 lab |
Virtual |
Applied PICO search strategies in medical databases and libraries |
Medical Informatics - MCS |
10 |
|
10 3.2 |
Date of Week 2 lab |
Virtual |
Documented a literature search using bibliographic records and Vancouver style references |
Medical Informatics - MCS |
11 |
10.6. Applies EBM principles in fundamental research and clinical practice
|
No. |
Date |
Patient |
Diagnosis and synthetic description |
Hospital |
Coordinator |
|
10.6.1 |
Date of Week 3 lab |
Virtual |
Completed a research protocol for a case-control study + Data analysis and interpretation of results. |
Medical Informatics - MCS |
12 |
|
10.6.2 |
Date of Week 5 lab |
Virtual |
Completed a research protocol for evaluating diagnostic and screening tests + Data analysis and interpretation of results. |
Medical Informatics - MCS |
13 |
|
10.6.3 |
Date of Week 8 lab |
Virtual |
Completed a research protocol for a survival study + Data analysis and interpretation of results. |
Medical Informatics - MCS |
14 |
|
10.6.4 |
Date of Week 6 lab |
Virtual |
Completed a research protocol for a randomized controlled trial + Data analysis and interpretation of results. |
Medical Informatics - MCS |
15 |
|
10.6.5. |
Date of Week 11 lab |
Virtual |
Choosing the appropriate statistical methods in different research scenarios |
Medical Informatics - MCS |
16 |
SECȚIA ENGLEZĂ – Conf. Dr. Horațiu Colosi
|
No. |
Themes |
Educational Objectives (required concepts & skills) To validate this course, students should be able to: |
|
1 |
Introduction. Importance of Medical Research Methodology. Variability. |
Motivate and explain the need to study and understand the proper design and implementation of medical studies, as well as the rules of medical writing and proper dissemination of research results, in their future positions as research “producers” (graduation thesis researcher) and research “consumers” (medical practitioners). Define medical information, medical data and its processing (medical informatics). Define biological variability and medical variables. Classify medical variables by type. Exemplify the types of medical variables. Recognize the types of medical variables in specific cases. Explain ways of collecting accurate medical information and coding it as medical data. Explain correct ways of presenting and measuring medical data (measurement scales) and their interpretation as medical information. Collect and encode medical information correctly (transforms information to data properly). Correctly present medical data (reconstruct information from data properly). |
|
2 |
Sequences (phases) of medical research. Bibliographic documentation. |
Explain the sequences (stages/phases) of medical research. Explain the utility of bibliographic documentation in medical research and medical practice. Explain the stages of research in which bibliographic documentation is necessary. Exemplify commonly used bibliographic medical databases. Define and applies bibliographic search strategies using the PICO technique for specific situations. Use the computer for bibliographic documentation: applies bibliographic search strategies using the PICO technique in PubMed (MEDLINE). Formulate appropriate research questions. Associate correct hypotheses to relevant research questions. Applies search strategies to identify medical sources in the UMF Cluj Library. Choose relevant literature for specific research questions. List the components of a bibliographic catalog. Create a correct bibliographic catalog in electronic format on a given research question. Explain the utility of references in research. Explain and applies the Vancouver referencing system for different types of medical text (original article, book, etc.). Correctly write various types of references according to Vancouver system. |
|
3 |
Sequences (phases) of medical research. The research protocol. Study variables. Types of research. Research Team. |
Formulate the sequences (phases) of a medical study. Names and explains the composing parts of a medical research protocol /plan. Identify the components of the research protocol for specific research scenarios. Name and explain the types of data collection in medical studies. Name and explain the kinds of sampling (probabilistic and non-probabilistic) in medical studies. Formulate the goal and objectives for specific research scenarios. Formulate hypotheses for specific research scenarios. Correctly recognize the domain of clinical research. Recognizes and classifies medical studies by type (depending on the objectives and expected results). Correctly define the target population and accessible population of a medical study. Correctly formulate inclusion and exclusion criteria for a study sample in a given research scenario. Correctly identify the type of data collection for a given research scenario. List the methods for describing different types of variables in specific medical studies (through tables, graphs and descriptive statistics). Correctly apply methods for describing different types of variables in specific medical studies using statistical software (tables, graphs and descriptive statistics). Explain the meaning of descriptive results of concrete medical studies (tables, graphs and descriptive statistics). List correct methods of data analysis for different variable types in concrete medical studies (statistical tests, correlation, regression models, statistical and medical indicators). Apply correct methods of data analysis for different variable types in concrete medical studies using statistical software (statistical tests, correlation, regression models, statistical and medical indicators). Explain the meaning of specific analytical results of medical studies (tests, correlation, regression models, statistical and medical indicators). |
|
4 |
Descriptive studies. Studies evaluating prognostic factors of diseases. |
Explain descriptive studies as sources of new hypotheses. Explains descriptive research questions, as opposed to analytic research questions. Define the assessment (evaluation) studies of prognostic factors (involved in different pathologies). Explain different types of evaluation studies of prognostic factors. Explain the methodology of evaluation studies of prognostic factors. Construct contingency tables based on data from specific research scenarios of prognostic factors, or based on data files. Test the existence of links between prognostic factors and diseases. Calculate medical/health indicators to quantify the importance of links between prognostic factors and illnesses. Evaluates the existence of a causal link between the presence of prognostic factors and the development of diseases of interest. Choose and justifies suitable medical indicators for quantifying the relationship between prognostic factors and diseases. Statistically interpret medical indicators used to quantify the relationship between prognostic factors and diseases (point estimates and their 95% confidence intervals). Clinically interpret medical indicators used to quantify the relationship between prognostic factors and diseases (point estimates and their 95% confidence intervals). Assess the precision and clinical importance of these results. Discriminate between precision and accuracy / validity of results. |
|
5 |
Studies evaluating diagnostic methods / tests.
|
Define assessment studies of diagnostic tests. Explain the four phases in the evaluation of diagnostic tests. Explain the methodology for studies evaluating diagnostic tests. Construct contingency tables based on data from specific research scenarios of diagnostic tests, or based on data files. Test the existence of links between the results of compared diagnostic tests. Calculate medical indicators to quantify the qualities of diagnostic tests. Choose and justifies suitable medical indicators for measuring the qualities of diagnostic tests (screening /confirmation). Statistically interpret medical indicators used to quantify the qualities of diagnostic tests (point estimates and their 95% confidence intervals). Clinically interpret medical indicators used to quantify the qualities of diagnostic tests (point estimates and their 95% confidence intervals). Assess the precision and clinical importance of these results. Discriminate between precision and accuracy / validity of results. |
|
6 |
Studies evaluating therapeutic approaches / treatment options. |
Define assessment studies of treatment options. Explains different approaches in the evaluation of treatment options. Explain possible methodological approaches of experimental evaluation studies of therapeutic approaches (types of Clinical trials, Phases I, II, III, IV). Construct contingency tables based on data from specific research scenarios of clinical trials, or based on data files. Test the existence of links between the assessed therapeutic factors and the experimental events defined in the study; assessing the existence of a causal link between them. Calculate medical indicators to quantify therapeutic efficacy/safety. Choose and justifies suitable medical indicators for measuring therapeutic efficacy/safety. Statistically interpret medical indicators used to quantify therapeutic efficacy/safety (point estimates and their 95% confidence intervals). Clinically interpret medical indicators used to quantify therapeutic efficacy/safety (point estimates and their 95% confidence intervals). Assesses the precision and clinical importance of these results. Discriminate between precision and accuracy / validity of results. |
|
7 |
Survival analysis studies |
Define survival studies (time to event studies). Explain the main characteristics of survival variables. Explain the methodology of survival studies. Test the existence of links between prognostic factors and time/probability of survival. Calculate medical indicators to quantify the importance of links between prognostic factors and time/probability of survival. Choose and justify suitable medical indicators used to quantify the importance of links between prognostic factors and time/probability of survival. Statistically interpret medical indicators used to quantify the importance of links between prognostic factors and time/probability of survival (point estimates and their 95% confidence intervals). Clinically interpret medical indicators used to quantify the importance of links between prognostic factors and time/probability of survival (point estimates and their 95% confidence intervals). Assess the precision and clinical importance of these results. Discriminate between precision and accuracy / validity of results. |
|
8 |
Validity of the study. Evidence cutting errors in medical studies |
The student: Defines random errors. Defines systematic errors (bias). Defines and exemplifies confounders. Classifies and defines the main types of selection bias. Explains methods that can be used to prevent / reduce / control selection bias. Classifies and defines the main types of observation / information bias. Explains methods that can be used to prevent / reduce / control observation / information bias. Classifies and defines the main types of analysis bias. Explains methods that can be used to prevent / reduce / control analysis bias. Explains methods that can be used to prevent / reduce / control confounders. Explains and distinguishes between precision and accuracy of research results. Explains and distinguishes between validity and relevance of research results. Recognizes different types of bias in specific scenarios of medical studies. Explains the influence of different types of bias on study results in specific scenarios of medical studies. Recognizes confounders in specific scenarios of medical studies. Explains the influence of confounders on study results in specific scenarios of medical studies. |
|
9 |
Evidence-based medicine (EBM) |
Defines evidence-based medicine (EBM) and evidence-based research. Classifies types of studies in the hierarchy of evidence for the diagnostic, prognostic, prophylactic, therapeutic domains of research. Explains the criteria for assessing the validity of studies in the diagnostic, prognostic, prophylactic, therapeutic domains. Explains the relevance of study results in medical practice and medical research. Critically appraises evidence from literature on concrete examples of medical studies (risk or protective factors, diagnostic tests, therapeutic approaches). Critically appraises evidence from systematic reviews on concrete examples. Ranks literature search results (from PubMed) by the type of studies, according to the hierarchy evidence for each domain of clinical research: prognostic, diagnostic, therapy. Explains in medical terms (for peers) and critically assesses scientific results found in literature. Explains in lay terms (for patients) scientific results found in literature. |
|
10 |
Meta-analysis |
Defines the systematic review and meta-analysis. Explains the methodology for a systematic review with or without meta-analysis. Explains the problem of effect size. Explains the forest plot. Decides and justifies suitable indicators for meta-analysis of different types of tracked results (dichotomous, quantitative, survival). Statistically interprets a forest plot diagram (point estimate and 95% confidence interval). Clinically interprets the results of a meta-analysis (point estimate and 95% confidence interval). Evaluates the precision and validity of results of a meta-analysis. |
|
11 |
Choosing statistical methods |
Defines statistical tests. Explains point estimates and confidence intervals. Explains the benefits offered by confidence intervals over the results of statistical tests. Names the factors which determine the choice of statistical methods. Formulates the null and alternative hypotheses of a statistical test for specific scenarios of medical research. Identifies appropriate statistical methods to compare independent groups in specific situations of medical research. Identifies appropriate statistical methods for comparisons of dependent (paired) groups in specific situations of medical research. Identifies appropriate statistical methods to assess the relationship between two or more variables for specific situations of medical research. |
|
12 |
Medical writing and communicating research results |
Lists and describes the main types of medical scientific publications. Lists and explains the objectives and qualities of scientific medical writing. Lists and explains the principles of medical scientific writing. Describes the structure and chapters of a graduation thesis in medicine. Indicates and explains the content for each chapter of a graduation thesis in medicine. Describes the structure and chapters of an original research article in medicine. Indicates and explains the content for each chapter of an original research article in medicine. Explains the structure, content and drafting rules of an oral communication in support of medical research (article or graduation thesis). Explains the structure, content and drafting rules of a poster presentation. Explains and discusses the ethical principles governing scientific medical writing. Critically appraises in terms of structure and content an article or article fragment offered for reading and critical evaluation. Chooses the appropriate type of graphics for presentation of results according to the type and nature of the distribution of the data (pie charts, column charts, box and whiskers, histograms, scatter, Kaplan-Meier survival curves). Explains and applies the rules of correct scientific writing of research results in the form of text, tables and graphs. Produces the slides for an oral presentation of an article or thesis according to the rules of scientific medical writing. |
|
13 |
Research Ethics |
Lists important phases in the historical evolution of medical research ethics. Lists important declarations of ethical standards in medical research. Lists the composition and duties of a medical research ethics committee (REC). Lists the ethical principles that govern medical research. Explains and discusses the ethical principles that govern medical research. Lists and explains the kinds of fraud and misconduct in medical research and publication. Lists regulatory bodies in medical research and publication ethics and their specific tasks. Identifies and explains the need to apply ethical principles in the context of different types of concrete medical studies. Specifies procedures for applying ethical principles in the context of concrete medical research projects. |
|
14 |
Regression analysis and modelling in medical research |
Defines modeling and simulation in research and explains their purpose. Exemplifies models in various fields of medical research. Evaluates correlation between quantitative variables using correlation coefficients and scatter charts. Explains and interprets models by simple and multiple linear and logistic regressions. |
Exam validation and bonuses
In order to validate the exam, the marks obtained in both the practical and theoretical examinations need to be higher or at least equal to 5.
The practical examination has a weight of 30% in calculating the final mark.
If the practical score is less than 10 but higher than 5, the group tutor may award up to 1 bonus point for the student’s activity during the semester.
During both exams, students are allowed to consult a personal single page with hand-written notes, in order to encourage comprehension and discourage learning by heart. Example questions or questionnaires from previous years, printed or copied material, as well as books, are NOT allowed and will be considered exam fraud.
The written exam consists of 40 multiple choice questions to be solved in 80 minutes.
The multiple choice questions are weighted based on their difficulty, hence, the test will be scored out of 100 points.
The written exam has a weight of 70% towards the final mark.
Up to 1 bonus point may be awarded to students who interact constructively and repeatedly during lectures. The bonus may be added to the final mark of the written examination, but only if that mark is already above 5.
Correction method of the written exam:
- Question with one correct answer:
- 5 concordances = 1 point
- less than 5 concordances = 0 points
- Question with 2 correct answers:
- 5 concordances = 1 point
- 4 concordances = 0.8 points
- less than 4 concordances = 0 points
- Question with 3 or more correct answers:
- 5 concordances = 1 point
- 4 concordances = 0.8 points
- 3 concordances = 0.5 points
- less than 3 concordances = 0 points
Mandatory Bibliography
- Lecture presentations
- Learning material distributed during lectures
- All practical activities and their explanatory files, accessible on www.info.umfcluj.ro
Optional Bibliography
- Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TB. Designing Clinical Research. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013.
- Drugan T, Berghe AS, Bolboaca SD, Bondor C, Calinici C, Colosi H, Cutas A, Iancu M, Istrate D, Leucuta DC, Valeanu M. Metodologia Cercetării Științifice Medicale. Cluj-Napoca: Editura Medicală Universitară „Iuliu Haţieganu”, 2017.






